A Brief Exposure to Magnetic Distortions During Embryonic Development
May Compromise the Migration of Loggerhead Hatchlings
MICHAEL SALMON
Department of Biological Sciences, Florida Atlantic University,
Boca Raton, Florida, 33431, USA [salmon@fau.edu].
Boca Raton, Florida, 33431, USA [salmon@fau.edu].
ABSTRACT. – In a previous study it was shown that loggerhead eggs exposed during embryonic development to a distorted magnetic field produced hatchlings incapable of using magnetic information for orientation or navigation. My study was done to determine if hatchlings could recover from those effects when magnetic distortions were confined to shorter time periods (the first, second or last third of embryonic development). Under those conditions I found that orientation still failed and conclude that even a relatively short exposure to field distortions might potentially incapacitate hatchlings that use magnetic cues during offshore migration.
Loggerhead hatchlings (Caretta caretta) typically emerge from their nests on western North Atlantic oceanic beaches at night, and then crawl to the surf zone and swim offshore. They then migrate to nursery areas often located hundreds of kilometers distant from the nest site. Observations done over several years have confirmed that many turtles find those areas even though juveniles at this stage in their development are both small and weak swimmers. They succeed by swimming into, then utilizing, oceanic surface currents (gyres) that help transport them to distant nursery areas (Lohmann and Lohmann 2003). They also detect the inclination angle and intensity of the earth’s magnetic field, magnetic attributes that vary spatially and provide unique paired values across the migratory routes and habitats frequented by the turtles during their early ontogeny. That sensitivity makes possible a bi-coordinate navigation system used to determine position within the North Atlantic Gyre (Lohmann and Lohmann 2003; Putman et al. 2011), as well as progress toward nursery sites adjacent to the Azores, Canary and Madeira Islands.
This study was inspired by an experiment done by Fuxjager et al. (2014) designed to determine how loggerhead hatchlings develop the ability to use magnetic information. They placed magnets in close proximity to the eggs so that the embryos developed in a distorted magnetic field. They then determined how hatchlings that emerged from those nests responded to magnetic and other orientation cues required for successful migration. They found that hatchlings were able to orient from the nest to the surf zone (using visual cues) and from the surf zone to deeper water (using the orbital motion contained in oceanic surface waves) but were unable to orient when presented with magnetic cues. Thus, a hatchling’s ability to respond appropriately to magnetic cues required that embryonic development was completed in a natural magnetic environment.
A number of studies, however, have shown that many vertebrate species can develop normally even when exposed to deficient environments through developmental homeostasis (Alcock 2009), a process that acts as a buffer by partially or completely suppressing non-adaptive phenotypes. My study was done to determine if turtle embryos exposed to magnets for shorter and/or different periods during embryonic development might emerge from nests still capable of using magnetic cues for orientation.
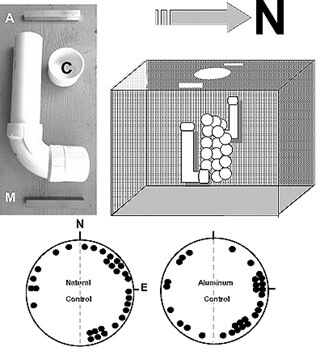
Methods. —To test this idea, I designed experiments in which two bar magnets, identical in size, shape and strength to those used by Fuxjager and associates (10-cm long x 1.4-cm wide), were placed on opposite sides of loggerhead nests during the first third (days 1–16; “early magnet”), second third (days 17–32; “middle magnet”) or last third (day 33 until emergence; “late magnet”) of the typical summer incubation period (47–50 days, depending upon temperature). A fourth group consisted of nests exposed to magnets for the entire incubation period (“total magnet” group) while a fifth group consisted of control nests exposed to a pair of (non-magnetic) aluminum bars (Fig. 1). A sixth (natural control) group consisted of undisturbed nests.
My study was carried out at Boca Raton, Florida, U.S.A.(26.22°N, 80.07°W) over three summers (July–Sept, 2016–2018). That lengthy time period was a consequence of two successively hot and dry seasons that reduced hatchling survival and compromised their vigor, especially for turtles that emerged from nests during the mid- to late summer. For all but the natural control nests, I constructed pairs of “J-shaped” PVC tubes (23-cm long x 5-cm outside diameter) and buried them ~30 cm to the east and west of the egg chamber, at a depth of ~ 1.0 m to the bottom (horizontal portion) of the tube (Fig. 1). Tubes were left in place from the morning after egg deposition until the end of incubation. I lowered bar magnets or aluminum bars into each tube by a string until they were positioned horizontally in a N-S direction at the bottom of the tube; the string was again used to retrieve the bars at an appropriate time. Otherwise, each tube was sealed with a cap (Fig. 1). Three nests were assigned to each of the 6 groups. They were established at the beach in a rotating sequence at intervals of 1–4 days. In the afternoon before an anticipated emergence, I removed a sample of hatchlings from each nest and placed them on a shallow layer of damp sand inside a small styrofoam™ cooler. The cooler was stored in a dark non-air conditioned laboratory until the evening when the responses of 10 hatchlings from each nest were recorded. All of the turtles were then released.
I determined the ability of each turtle to orient when only magnetic cues were available in an outdoor water-filled, circular polyethylene pool, 1.45 m in diameter x 53 cm tall, filled to a depth of 40 cm with fresh water. The pool was elevated on concrete blocks at a location where the horizontal and vertical magnetic components at the beach were closely approximated. The pool was covered during testing to eliminate stray lighting.

This system is pictured elsewhere (Merrill and Salmon 2011) and will only briefly be described here. Each turtle was placed in a cloth harness (Fig. 2) attached by a monofilament line to a lever arm. The arm rotated horizontally just above the water surface to track the turtle’s directional swimming movements. A digital encoder linked to the lever arm was used to record the turtle’s orientation and send that information to a computer. A dim light, located on one side of the tank, attracted the turtle so that it swam east during a 10 min “pretest” period. That response is used to activate the turtle’s magnetic orientation system. The light was then extinguished so that the turtle swam in total darkness during a 3-min “acclimation” period. The acclimation period was followed by a “test” period when the turtle’s heading was recorded every 10 sec for 5 min. Those 30 headings stored on computer were averaged to yield a mean heading for that subject.
Loggerhead hatchlings exposed to this testing regime (developed by Lohmann and Lohmann 2004) will orient in directions appropriate to the magnetic information they detect during the test period. In this experiment, that information was the inclination angle and intensity of the magnetic field at Boca Raton, Florida. The predicted response was an eastward heading, provided that the turtles had developed the capacity to respond to magnetic cues. To find out if they had, I compared the number of mean headings that were generally eastward (between 0° and 179°) to the number that were generally westward (180° through 359°) for the turtles under each treatment condition. A binomial test was used to determine if those headings were distributed randomly or if the turtles exhibited an eastward bias.
Results and Discussion. — While the turtles in the two control groups showed a significant eastward preference, all of the turtles exposed during development to a magnetically distorted field, regardless of when or for how long during incubation that exposure occurred, showed a distribution of mean headings that was indistinguishable from random (Table 1; Fig. 1). It appears that even a short exposure during embryogenesis (relative to the entirety of the incubation period) can affect the ability of hatchlings to utilize magnetic information. Whether that deficiency compromises the function of the magnetic compass, the ability to discern spatial position, or both capabilities is unknown (Fuxjager et al. 2014). It also remains to be determined whether turtles can eventually recover from these effects, and if so whether that recovery occurs more rapidly when the exposure period is shortened.
My results also suggest that nests placed near to structures containing magnetic materials (e.g., steel reinforced concrete groins or steel-containing seawalls) or nests exposed for shorter time periods to galvanized metal screens and/or cages, might produce hatchlings that appear normal as they can locate the sea from the nest and initially swim offshore, but in reality are incapacitated because they are unable to use magnetic maps to determining their present oceanic location or to orient toward distant nursery areas. Irwin et al. (2004) presented similar warnings.
Finally, there is little understanding of what developmental processes are affected. Rearing juvenile steelhead trout in a distorted magnetic field disrupts their ability to use magnetic maps (Putman et al. 2014). Exposure to magnetic disturbances even earlier in development affects the orientation of the embryo’s body inside the egg. This response, which indicates that embryos can detect and respond to the field, has been shown in fish (Formici and Winniki 1998) and in loggerhead embryos (Carthy et al. 2018). But how such a change in embryonic body orientation is related to the ability of juvenile fishes or turtles to use magnetic information remains an interesting, but currently unexplored, question. Hopefully, at some future date, additional experiments will provide an answer.
Acknowledgements. — This study was supported by personal funds and contributions from the National Save the Sea Turtle Foundation. Thanks to Kirt Rusenko and David Anderson of the Gumbo Limbo Environmental Complex for providing access to the nests on Boca Raton beach, and for allowing me to collect a sample of hatchlings from those nests. Gillian Davis provided inside space for a laboratory and a magnetically uniform outside patio for the test tank. Critical reading by Mary Jane Saunders, Jeanette Wyneken and Nathan Putman improved manuscript clarity and organization. The study design was peer-reviewed and subsequently permitted by the Florida Fish and Wildlife Conservation Commission (Turtle Permit-173).
LITERATURE CITED
ALCOCK, J.A. 2009. Animal Behavior, an Evolutionary Approach. Ninth Edition. Sunderland, MA: Sinauer Associates, Inc, 606 pp.
CARTHY, R.R., BROTHERS, J.R., BÉZY, V., LOHMANN, K.J., AND LAMONT, M.M. 2018. Ontogeny of orientation responses in developing sea turtle embryos. Paper presented at the International Sea Turtle Symposium, Kobe, Japan.
FORMIKI, K. AND WINNICKI, A. 1998. Reactions of fish embryos and larvae to constant magnetic fields. Italian Journal of Zoology 65:479–482.
FUXJAGER, M.J., DAVIDORF, K.R., MANGIAMELE, L.A., AND LOHMANN, K.J. 2014. The geomagnetic environment in which sea turtle eggs incubate affects subsequent magnetic navigation behaviour of hatchlings. Proceedings of the Royal Society B 281:1–8.
IRWIN, W.P., HORNER, A.J., AND LOHMANN, K.J. 2004. Magnetic field distortions produced by protective cages around sea turtle nests: Unintended consequences for orientation and navigation? Biology of Conservation 118:117–120.
LOHMANN, K.J. AND LOHMANN, C.M.F. 2003. Orientation mechanisms of hatchling loggerheads. In: Bolten, A.B. and Witherington, B.E. (Eds.) The Loggerhead Sea Turtle. Smithsonian Books, Washington, D.C. pp. 44–62.
LOHMANN, K.J. AND LOHMANN, C.M.F. 1994. Detection of magnetic inclination angle by sea turtles: a possible mechanism for determining latitude. Journal of Experimental Biology 194:23–32.
MERRILL, M.M. AND SALMON, M. 2010. Magnetic orientation by hatchling loggerhead sea turtles (Caretta caretta) from the Gulf of Mexico. Marine Biology 158:101–112.
PUTMAN, N.F., ENDRES, C.S., LOHMANN, C.M.F., AND LOHMANN, K.J. 2011. Longitude perception and bicoordinate magnetic maps in sea turtles. Current Biology 21:463–466.
PUTMAN, N.F., MEINKE, A.M., AND NOAKES, D.L. 2014. Rearing in a distorted magnetic field disrupts the "map sense" of juvenile steelhead trout. Biology Letters 10(6): 20140169.
http://rsbl.royalsocietypublishing.org/content/10/6/20140169.short.
CARTHY, R.R., BROTHERS, J.R., BÉZY, V., LOHMANN, K.J., AND LAMONT, M.M. 2018. Ontogeny of orientation responses in developing sea turtle embryos. Paper presented at the International Sea Turtle Symposium, Kobe, Japan.
FORMIKI, K. AND WINNICKI, A. 1998. Reactions of fish embryos and larvae to constant magnetic fields. Italian Journal of Zoology 65:479–482.
FUXJAGER, M.J., DAVIDORF, K.R., MANGIAMELE, L.A., AND LOHMANN, K.J. 2014. The geomagnetic environment in which sea turtle eggs incubate affects subsequent magnetic navigation behaviour of hatchlings. Proceedings of the Royal Society B 281:1–8.
IRWIN, W.P., HORNER, A.J., AND LOHMANN, K.J. 2004. Magnetic field distortions produced by protective cages around sea turtle nests: Unintended consequences for orientation and navigation? Biology of Conservation 118:117–120.
LOHMANN, K.J. AND LOHMANN, C.M.F. 2003. Orientation mechanisms of hatchling loggerheads. In: Bolten, A.B. and Witherington, B.E. (Eds.) The Loggerhead Sea Turtle. Smithsonian Books, Washington, D.C. pp. 44–62.
LOHMANN, K.J. AND LOHMANN, C.M.F. 1994. Detection of magnetic inclination angle by sea turtles: a possible mechanism for determining latitude. Journal of Experimental Biology 194:23–32.
MERRILL, M.M. AND SALMON, M. 2010. Magnetic orientation by hatchling loggerhead sea turtles (Caretta caretta) from the Gulf of Mexico. Marine Biology 158:101–112.
PUTMAN, N.F., ENDRES, C.S., LOHMANN, C.M.F., AND LOHMANN, K.J. 2011. Longitude perception and bicoordinate magnetic maps in sea turtles. Current Biology 21:463–466.
PUTMAN, N.F., MEINKE, A.M., AND NOAKES, D.L. 2014. Rearing in a distorted magnetic field disrupts the "map sense" of juvenile steelhead trout. Biology Letters 10(6): 20140169.
http://rsbl.royalsocietypublishing.org/content/10/6/20140169.short.
Submitted: 29 November 2018
Revised and Accepted: 20 February 2019
Handling Editor: Jeffrey A. Seminoff
Revised and Accepted: 20 February 2019
Handling Editor: Jeffrey A. Seminoff
Figure Legends
Figure 1. Above, left: a PVC tube is used to secure either a bar magnet (M) or a (control) aluminum bar (A) on each side of a sea turtle nest (above, right) placed on the beach at Boca Raton, Florida, U.S.A. Tubes are buried ~ 1 m below the sand surface and ~ 30 cm from the egg chamber. They remain in place throughout incubation. Bars are lowered into (and recovered from) the tube using a string. See the text for a description of the temporal schedule of bar placement. The tube is otherwise sealed with a cap (C). Circle diagrams (below) show the distribution of mean angles eastward vs. westward for the 30 hatchlings from three natural (undisturbed) and three aluminum control nests. In both groups most of the turtles swim toward the eastern half of the tank. See Table 1 for details and statistics.
Figure 2. A loggerhead hatchling, attached by a cloth harness to a lever arm, swims in a circular pool where its orientation abilities are measured. (M. Salmon photo)
Table 1. Distribution of mean orientation angles shown by loggerhead hatchlings swimming in complete darkness after orienting toward an east light for 10 minutes. Data are based upon the responses of 30 turtles (n = 10 turtles/nest from 3 different nests) that hatched from eggs exposed to one of the six treatments. Vectors chosen by each turtle are grouped by whether they fell within the eastern (0° - 179°) or the western (180° - 359°) half of the pool (See Figure 1). A binomial test (1 tailed) is used to determine if the distributions differ from random (at p < 0.05).
Treatments
Number of turtles swimming:
Binomial Probability
(3 nests in each)
Eastward
Westward
(1 tailed)
Natural Control
23
7
0.003
Aluminum Control
21
9
0.02
Total Magnet
17
13
n.s.
Early Magnet
14
16
n.s.
Middle Magnet
14
1
n.s.
Late Magnet
17
13
n.s.
Helping Sea Turtles Survive for 39 Years
A NON-PROFIT ORGANIZATION
State of Florida Registration Number CH-2841 | Internal Revenue Code 501 (c) (3)
Web Design & Development by Web Expressions, LLC

