Experimental Analysis of Wavelength Preferences Shown by Hatchling Leatherback Sea Turtles (Dermochelys coriacea)
Article in Chelonian Conservation and Biology · October 2022

Samantha Trail
Florida Atlantic University

Michael Salmon
Florida Atlantic University
Chelonian Conservation and Biology, 2022, 21(2): 283–286 doi:10.2744/CCB-1535.1 © 2022 Chelonian Research Foundation
Experimental Analysis of Wavelength Preferences Shown by Hatchling Leatherback Sea Turtles (Dermochelys coriacea)
SAMANTHA E. TRAIL* AND MICHAEL SALMON
Department of Biological Sciences, Florida Atlantic University, Boca Raton, Florida 33431-0991 USA [strail2019@fau.edu; salmonmichael07@gmail.com] *Corresponding author
ABSTRACT. - In marine turtles it is well established that the shorter light wavelengths in the visible and near- ultraviolet spectrum provide more potent, and pre- ferred, cues for nocturnal seafinding orientation than the longer light wavelengths. In this study, we simultaneously presented leatherback hatchlings (Der- mochelys coriacea) with a short near-ultraviolet (380 nm) and a longer visible (500 nm) light stimulus to determine whether that preference was based upon differences in light intensity, light wavelength, or a combination of both variables. We found that under light conditions mimicking those at the nesting beach on the darkest evenings, the behavioral preference for the shorter light wavelengths was based upon intensity cues, though we speculate that under brighter illumi- nation, wavelength cues might also be utilized.
Marine turtles crawl up the beach to nest, generally at night. They dig an egg chamber where they deposit 50– 200 eggs (depending upon species) before covering the nest with sand and returning to the sea. After completing development, the embryos break out of the egg and, as hatchlings, dig their way upward until they emerge from the nest. Emergence usually occurs at night and is followed almost immediately by an oriented crawl from the nest to theocean — an innate behavior known as ‘‘seafinding’’ (Lohmann et al. 1997).
Evidence gathered since the 1940s has established that seafinding is mediated by visual cues (Daniel and Smith 1947) and takes the form of a positive phototaxis; the turtles crawl towards the lowest and brightest (that is, most light-radiant) horizon, generally located in a seaward direction (Mrosovsky and Shettleworth 1968; Limpus 1971). The wavelengths they detect emanate from celestial sources (stars, moon) and consist of both visible and near- ultraviolet (near-UV) portions of the electromagnetic spectrum. Behavioral experiments have revealed that the wavelengths used by the hatchlings during seafinding range between 340 and 600 nm (Celano et al. 2018).
Previous studies have also demonstrated that the turtles are more strongly attracted to the shorter than to the longer light wavelengths (Mrosovsky and Carr 1967; Ehrenfeld 1968; Mrosovsky and Shettleworth 1968; Witherington and Bjorndal 1991; Witherington 1992), leading to the assertion that these light stimuli are ‘‘preferred.’’ Why marine turtle hatchlings should show such a bias is not at all obvious. One possibility is that by doing so, they can more accurately discriminate differ- ences shown between the landward and seaward horizon. Recent measurements have shown that these horizons vary quantitatively in radiance (the seaward view is generally brighter), but not qualitatively in light spectra (Celano et al. 2018). Nevertheless, this issue remains an intriguing puzzle that requires further investigation.
A second feature of the orientation response, and one we consider in this study, centers on how that preference is determined when it most commonly occurs: at night. In this paper we explored the possibilities that the preference was based upon differences in light intensity (radiance), light wavelength, or some combination of both variables. Similar designs have been used to test whether sea turtles are capable of discerning color (Young et al. 2012). On the basis of our experimental results, we conclude that most commonly at night light intensity, not wavelength, is the primary cue used by hatchlings to orient in a seaward direction.
Methods. — The methods used in this study have been described in 2 publications (Celano et al. 2018; Trail and Salmon 2022) and will be only briefly summarized here. We obtained leatherback (Dermochelys coriacea) hatch- ings from nests monitored between May and September 2020, at 2 nesting beaches in southeastern Florida: Juno Beach (lat 26.888N, long 80.058W) and Boca Raton (lat 26.378N, long 80.138W). All of the hatchlings were collected from each nest in the late afternoon of the evening that they would naturally emerge. They were then transported to our lab on the campus of Florida Atlantic University, used for tests that night, then immediately released at the Boca Raton nesting beach.
Experiments were done using a black PlexiglasTM Y- maze to simultaneously present the hatchlings with a short, near-UV (380 nm) and a longer, visible (500 nm) light wavelength stimulus. Each light was projected from the end of one maze arm (Fig. 1) and each induced a positive phototaxis. A preference for one light over the other was measured by the distribution of crawl entries into each arm.
To determine if that preference was based upon light intensity or light wavelength, it was necessary to control for differences in stimulus intensity. That was done by using threshold data determined previously at each of the 2 chosen wavelengths (Fig. 2, upper graph; Trail and Salmon 2022). That threshold is defined as the dimmest light stimulus that evoked a statistically significant preference for one arm illuminated by that wavelength over the other arm, lacking any light stimulus. Each of the paired lights was then presented to the turtles at an identical quantal intensity (0.3-, 0.7- or 1.0-log unit) above threshold, and at radiance values that might duplicate those measured at the beach under new moon conditions. Thus, if the preference was based upon intensity cues, then the turtles should show a random (near 50:50) distribution of arm entries. However, if the preference was based upon light wavelength, then the turtles should prefer one arm (predictably, the one presenting the shorter, near-UV wavelength) over the other.
CHELONIAN CONSERVATION AND BIOLOGY , Volume 21, Number 2 – 2022
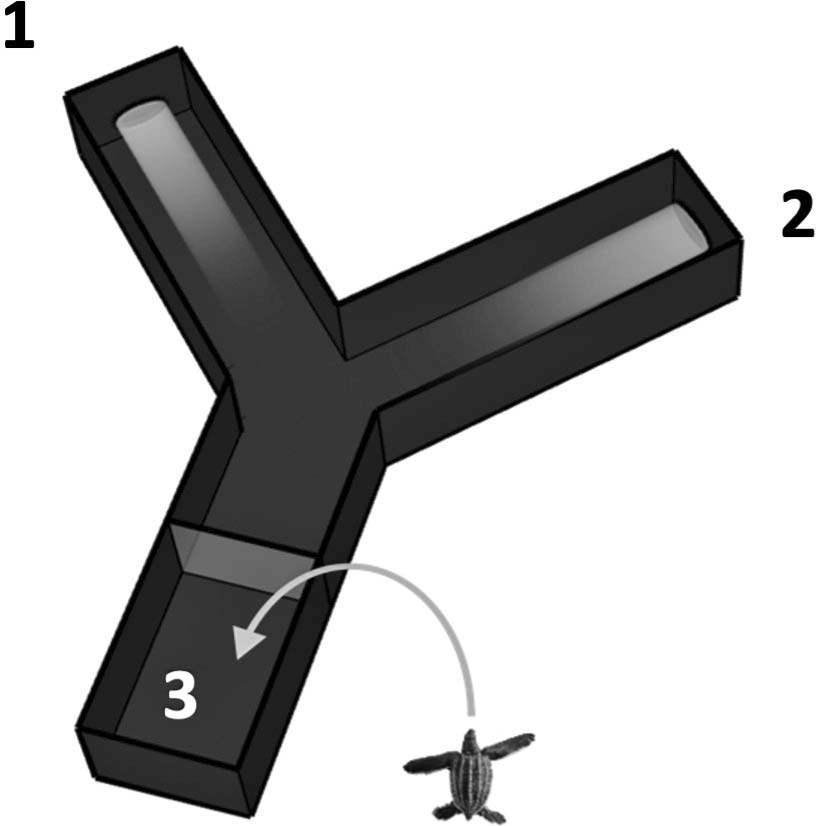
Figure 1. The Y-maze system used to determine wavelength preferences. (1) near-ultraviolet wavelength (380 nm); (2) visible wavelength (500 nm); (3) start area. The Y-maze measures 38 3 15 cm (length 3 width) within the start area and the split, and 41 3 13 cm within each arm.
The 500-nm light stimulus was projected at the end of one arm as a circular target, 6 cm in diameter, using a Kodakt slide projector (Model 440) containing a 300-W tungsten halogen lamp. The 380-nm stimulus was projected at the end of the other arm using a Great Value TM 14-W fluorescent ‘‘blacklight’’ bulb (Model EDXO-14), placed inside a foil-lined, 21-l Styrofoam TM box containing a 6-cm circular hole positioned against the arm end. Interference (5-nm half band width) and neutral density filters (Edmund Scientific Optics, Blackwood, NJ; 25-mm diameter) were used to obtain the desired wavelength and radiance, excluding any variation that might occur due to voltage fluctuations. Each stimulus was confirmed to be within 6 3 nm of its designated wavelength by spectrometer measurements (SRI 2000, Allied Scientific Pro, Gatineau, Quebec, Canada). Intensity measurements were also verified regularly between trials and adjusted as necessary. Stimulus intensities were measured in W/sec using a UDT S471 Optometer (UDT, San Diego, CA), calibrated for use with a UDT 247 sensor for nearly equivalent sensitivity to wavelengths between 340 and 600 nm. Values were then converted into photons/ cm 2 /sec.
Hatchlings were initially dark-adapted, then placed in the starting area of the Y-maze where they were briefly prevented from crawling ahead by a clear plastic barrier. Once that barrier was lifted, each hatchling had 5 min to crawl into one or the other arm. Between trials with different clutches, excess sand was removed and the maze was cleaned with 0.05% chlorohexidine solution. Up to 14 hatchlings (depending upon availability), each used only once, were tested to obtain an informative distribution of arm entries based upon a 1-tailed binomial test (at p ≤ 0.05; Zar 1999).
Results and Discussion. — A total of 41 hatchlings from 7 nests, tested at each of the 3 perceptually equivalent paired intensities, showed no statistically significant preference for either arm (Table 1). That outcome indicated their choices were based upon the detection of what they perceived as equivalent radiances. These data also showed that because the turtles performed so consistently, only a small number of trials were required to obtain statistically significant results (Celano et al. 2018; Trail and Salmon 2022). We conclude that the preference for the shorter wavelengths, demonstrated by many others, was based upon differences in stimulus radiance. Given that the turtles are more sensitive to the shorter light wavelengths (Fig. 2), it is likely that at the nesting beach those stimuli were perceived as brighter than the longer light wavelengths.
Different conclusions were reached in earlier studies carried out by Mrosovsky and Carr (1967) and Mrosovsky and Shettleworth (1968), working with green turtles (Chelonia mydas). They concluded that the preference was based upon both intensity and wavelength cues. However, the evidence for wavelength discrimination was based upon the assumption that physiological and behavioral thresholds for light were concordant. That assumption was subsequently proven false (see below).
Specifically, Mrosovsky and Shettleworth (1968) presented hatchlings with a choice between crawling toward 2 light sources presented simultaneously: a red (long wavelength) and a blue (short wavelength) stimulus. As expected, the blue stimulus was initially preferred, but that preference was reversed when the intensity of the red light was 2.8 log units brighter than the intensity of the blue light. Mrosovsky and Shettleworth (1968) noted that the blue light threshold (as measured physiologically from the retina by Granda and O’Shea 1972) was 2 log units lower than the red light threshold. On that basis, they concluded that the ‘‘...0.8 log unit discrepancy between the brightness estimated by the ERG method and the brightness estimated by the behavioral method...’’ implied that both light intensity and light wavelength governed the preference. But in marine turtles, spectral sensitivities measured physiologically (Fig. 2, lower graph) differ from those measured perceptually (Celano et al. 2018; Trail and Salmon 2022) and so the 2 sources of data are not comparable.
NOTES AND FIELD REPORTS
Table 1. Distribution of Y-maze arm entries by leatherback hatchlings when presented with 380-nm and 500-nm light stimuli. Each wavelength was projected from one arm of the Y-maze at the same time, and at perceptually equivalent intensities relative to the threshold at that wavelength. Under those conditions, the turtles showed no statistical preference for the shorter light wavelength (by a 1-tailed binomial test).
Response distribution
Log intensity above threshold
380 nm
500 nm
No. of hatchlings tested
p
0.3
0.7
1.0
Totals:
0.7
1.0
Totals:
9
4
8
21
4
8
21
5
9
6
20
9
6
20
14
13
14
41
13
14
41
0.21
0.13
0.40
-
0.13
0.40
-
These experiments and resulting conclusions were made possible by obtaining information that was previ- ously unavailable, specifically, detailed measurements of the light spectra present at night, tests to determine which wavelengths elicited a positive phototaxis, and thresholds of the hatchlings to those wavelengths (Celano et al. 2018; Trail and Salmon 2022). This approach was long overdue as previous demonstrations that the turtles preferred the shorter wavelengths implied wavelength discrimination in the absence of relevant supporting data.
Our conclusions are also consistent with the condi- tions under which visual tasks are usually accomplished by most vertebrates under nocturnal lighting conditions. At night, vision is dominated by rods, which typically provide no information useful for color discrimination, especially in eyes that are designed for function (as are those of marine turtles) primarily under bright lighting conditions (Fritsches and Warrant 2013). However, if hatchlings emerge just before dusk (as often occurs in this species; Gonzales and Stewart 2019) or under full moon illumina- tion, both rods and cones might be activated. Under those conditions, Mrosovsky and Shettleworth (1968) could be correct — color might also contribute to a phototaxis preference.
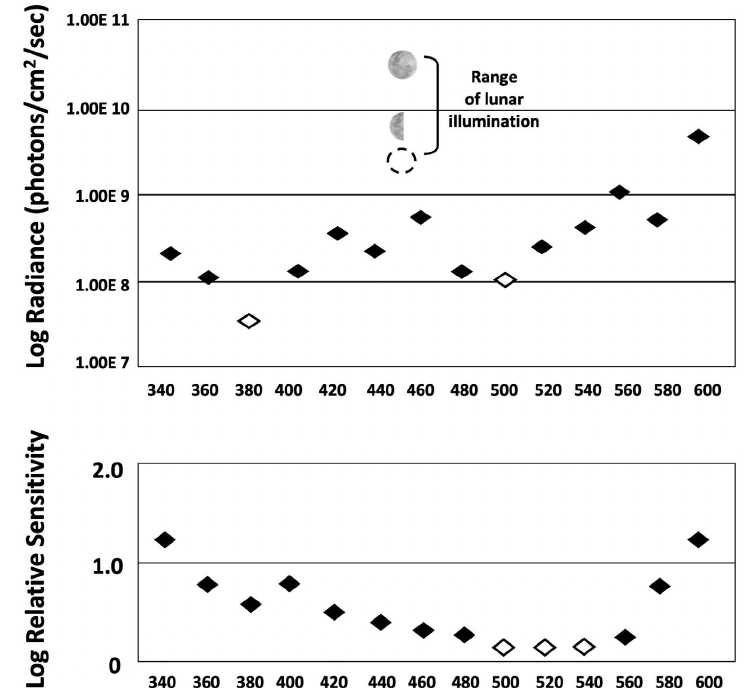
Figure 2. Above, spectral sensitivity of leatherback hatchlings to near-monochromatic light wavelengths (k) measured behaviorally as phototaxis thresholds relative to the nocturnal light radiance present at a (Juno Beach) Florida nesting site, and (below) physiologically as ERG response thresholds recorded from the retina. The 2 distributions differ in the following respects: First, the range of threshold values is greater behaviorally (. 2 log units) than physiologically (, 2 log units). Second, the wavelengths detected with the greatest sensitivity (open diamonds) are among the shortest behaviorally and among the longest physiologically. (Above, from Trail and Salmon [2022]; below, modified from Horch et al. [2008]).
Acknowledgments.— This study was completed by S.E.T. in partial fulfillment of the requirements for a Master of Science degree in the Department of Biological Sciences at Florida Atlantic University (FAU). We thank M.J. Saunders, J. Seminoff, and 2 anonymous referees for comments that improved manuscript organization and clarity. The project was supported by the department and by the National Save the Sea Turtle Foundation of Fort Lauderdale, Florida. The study was authorized by the State of Florida (FWC Permit no. MTP-19-173A) and by the FAU animal care committee (IACUC Protocol A20-13).
LITERATURE CITED
CELANO, L., SULLIVAN, C., FIELD , A., AND S ALMON, M. 2018. Seafinding revisited: how hatchling marine turtles respond to natural lighting at a nesting beach. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural & Behavioral Physiology 204:1007–1015.
DANIEL, R.S. AND S MITH , K.U. 1947. The sea-approach of the neonate loggerhead turtle (Caretta caretta). Journal of Comparative Physiology and Psychology 40:413–420.
EHRENFELD, D.W. 1968. The role of vision in the sea-finding orientation of the green turtle (Chelonia mydas). Animal Behaviour 16:281–287.
FRITSCHES, K.A. AND WARRANT , E.J. 2013. Vision. In: Wyneken, J., Lohmann, K.J., and Musick, J.A. (Eds.). The Biology of Sea Turtles, Vol 3. Boca Raton, FL: CRC Press, pp. 32–53.
GONZALES, C.M. AND STEWART, K.R. 2019. Emergence timing of leatherback hatchlings (Dermochelys coriacea) at Sandy Point National Wildlife Refuge, 2010–2014. Chelonian Conservation and Biology 18:241–248.
GRANDA , A.M. AND O’S HEA , P.J. 1972. Spectral sensitivity of the green turtle (Chelonia mydas mydas) determined by electrical responses to heterochromatic light. Brain, Behavior, and Evolution 5:143–154.
DANIEL, R.S. AND S MITH , K.U. 1947. The sea-approach of the neonate loggerhead turtle (Caretta caretta). Journal of Comparative Physiology and Psychology 40:413–420.
EHRENFELD, D.W. 1968. The role of vision in the sea-finding orientation of the green turtle (Chelonia mydas). Animal Behaviour 16:281–287.
FRITSCHES, K.A. AND WARRANT , E.J. 2013. Vision. In: Wyneken, J., Lohmann, K.J., and Musick, J.A. (Eds.). The Biology of Sea Turtles, Vol 3. Boca Raton, FL: CRC Press, pp. 32–53.
GONZALES, C.M. AND STEWART, K.R. 2019. Emergence timing of leatherback hatchlings (Dermochelys coriacea) at Sandy Point National Wildlife Refuge, 2010–2014. Chelonian Conservation and Biology 18:241–248.
GRANDA , A.M. AND O’S HEA , P.J. 1972. Spectral sensitivity of the green turtle (Chelonia mydas mydas) determined by electrical responses to heterochromatic light. Brain, Behavior, and Evolution 5:143–154.
HORCH, K.W., GOCKE , J.P., SALMON , M. AND FORWARD, R.B. 2008. Visual spectral sensitivity of hatchling loggerhead (Caretta caretta L.) and leatherback (Dermochelys coriacea L.) seaturtles, as determined by single-flash electroretinography. Marine and Freshwater Behaviour and Physiology 41:79–91.
LIMPUS, C.J. 1971. Sea turtle ocean finding behaviour. Search 2: 385–387.
LOHMANN , K.J., W ITHERINGTON , B.E., LOHMANN , C.M., AND SALMON, M. 1997. Orientation, navigation, and natal beach homing in sea turtles. In: Lutz, P.L. and Musick, P.A. (Eds.). The Biology of Sea Turtles. Boca Raton, FL: CRC Press, pp. 107–136.
MROSOVSKY, N. AND CARR , A. 1967. Preference for light of short wavelengths in hatchling green sea turtles, Chelonia mydas, tested on their natural nesting beaches. Behaviour 28:217–231.
by MROSOVSKY , N. AND SHETTLEWORTH , S.J. 1968. Wavelength preferences and brightness cues in the water finding behaviour of sea turtles. Behaviour 32:211–257.
TRAIL , S.E. AND SALMON , M. 2022. Differences in visual perception are correlated with variation in sea-finding behaviour between hatchling leatherback(Dermochelys cor- iacea)and loggerhead (Caretta caretta) marine turtles. Animal Behaviour 187:47–54.
WITHERINGTON, B.E. 1992. Behavioral responses of nesting turtles to artificial lighting. Herpetologica 48:31–39.
WITHERINGTON, B.E. AND BJORNDAL, K.A. 1991. Influences of wavelength and intensity on hatchling sea turtle phototaxis: implications for sea-finding behavior. Copeia 1991:1060– 1069.
YOUNG , M., SALMON , M., AND FORWARD , R. 2012. Visual wavelength discrimination by the loggerhead turtle, Caretta caretta. Biological Bulletin (Woods Hole) 222:46–55.
ZAR, J.H. 1999. Biostatistical Analysis. Fourth edition. Hoboken, NJ: Prentice-Hall.
LIMPUS, C.J. 1971. Sea turtle ocean finding behaviour. Search 2: 385–387.
LOHMANN , K.J., W ITHERINGTON , B.E., LOHMANN , C.M., AND SALMON, M. 1997. Orientation, navigation, and natal beach homing in sea turtles. In: Lutz, P.L. and Musick, P.A. (Eds.). The Biology of Sea Turtles. Boca Raton, FL: CRC Press, pp. 107–136.
MROSOVSKY, N. AND CARR , A. 1967. Preference for light of short wavelengths in hatchling green sea turtles, Chelonia mydas, tested on their natural nesting beaches. Behaviour 28:217–231.
by MROSOVSKY , N. AND SHETTLEWORTH , S.J. 1968. Wavelength preferences and brightness cues in the water finding behaviour of sea turtles. Behaviour 32:211–257.
TRAIL , S.E. AND SALMON , M. 2022. Differences in visual perception are correlated with variation in sea-finding behaviour between hatchling leatherback(Dermochelys cor- iacea)and loggerhead (Caretta caretta) marine turtles. Animal Behaviour 187:47–54.
WITHERINGTON, B.E. 1992. Behavioral responses of nesting turtles to artificial lighting. Herpetologica 48:31–39.
WITHERINGTON, B.E. AND BJORNDAL, K.A. 1991. Influences of wavelength and intensity on hatchling sea turtle phototaxis: implications for sea-finding behavior. Copeia 1991:1060– 1069.
YOUNG , M., SALMON , M., AND FORWARD , R. 2012. Visual wavelength discrimination by the loggerhead turtle, Caretta caretta. Biological Bulletin (Woods Hole) 222:46–55.
ZAR, J.H. 1999. Biostatistical Analysis. Fourth edition. Hoboken, NJ: Prentice-Hall.
Received: 20 December 2021 Revised and Accepted: 10 May 2022 Handling Editor: Jeffrey A. Seminoff
Helping Sea Turtles Survive for 39 Years
A NON-PROFIT ORGANIZATION
State of Florida Registration Number CH-2841 | Internal Revenue Code 501 (c) (3)
Web Design & Development by Web Expressions, LLC

