Black Saltmarsh Mosquitos - Things That Hum In The Night
David S. Addison
Conservancy of Southwest Florida
Naples, Florida 34116
Conservancy of Southwest Florida
Naples, Florida 34116
Scott Alexander Ritchie, Ph.D.
James Cook University
Cairns Queensland 4870, Australia
James Cook University
Cairns Queensland 4870, Australia

Fig.3. Flooded pond in mangrove basin forest. The larvae
and pupae congregate in large assemblages called “rafts”.
The presence of rafts can be detected because the larval
activity makes water’s surface shimmer.
and pupae congregate in large assemblages called “rafts”.
The presence of rafts can be detected because the larval
activity makes water’s surface shimmer.
Florida’s fame as a mosquito factory goes back to the 1500’s when Spanish explorers dubbed the entire east coast “Los Mosquitos”. Their numbers did not diminish over time either. In 1824, the area between Volusia and Palm Beach Counties was designated as Mosquito County. Florida’s southwest coast was equally infested if not more so. They were so ubiquitous that people mostly avoided the region until the late 19th century. Colloquially, they were referred to as the, “Defender of the Everglades”.
Anybody who has conducted night surveys on sea turtle nesting beaches, especially in SW Florida, can tell you that, in spite of habitat loss and modern abatement measures, they are still around in what at times are stupefying numbers. How is this so? Thirty years ago a colleague (an entomologist) and I studied the ecology of the black saltmarsh mosquito (Aedes taeniorhynchus). We spent two years trudging about in the mangrove forests of SW Florida as we studied the ways of this oh so successful species.
A. taeniorhynchus or “Taenies” for short, are a floodwater species that depends on fluctuations in water levels to drive their population dynamics. Florida’s 1,300 miles of coastline, flat topography and subtropical climate work in concert to create what amounts to an ecosystem made in heaven for Taenies. Those who have conducted field work in South Florida’s coastal ecosystems appreciate better than most just how well Taenies utilize their environment. So just how does this all work to produce so many mosquitos?
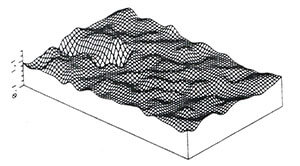
Fig. 2. Surface contour plot showing the
subtle changes in the topography of the
floor of a mangrove basin forest. Tic marks
on left are at 6.1 cm intervals. The study
plots were is 6.1 m x 30.5 m.
subtle changes in the topography of the
floor of a mangrove basin forest. Tic marks
on left are at 6.1 cm intervals. The study
plots were is 6.1 m x 30.5 m.
For starters, Taenies lay their eggs in moist soil where they can remain viable for months. The females lay about 25 eggs without a blood meal, however, with a blood meal they produce 50-200 eggs. The eggs hatch when the substrate floods. They are aggressive biters and will take a blood meal from any available source (Fig. 1). In South Florida, the fringes of the mangrove forests are regularly flooded by tides so their eggs don’t accumulate in great numbers, and most of the larvae that result are eaten by fish introduced by frequent tides. Further into the forest, there is typically a berm or natural levee that prevents inundation by all but the most extreme high tides. Forests with a lower berm are still frequently flooded by tides that introduce fish. However, forests with higher berms that contain potholes and depressions can become fish-free ponds when flooded by heavy rain (Fig. 2). These areas become ever dryer in late winter and during the dry season in spring. It is in these times of the year that their eggs accumulate in the soil. Sporadic rainfall triggers the intermittent production of broods of mosquitoes in these ponds. Taenies actively avoid oviposition in tidally flooded areas that contain predators such as fish, preferentially laying eggs in the higher, rain-flooded basins. The hatched eggs (or eggshells) persist in the substrate for years. By collecting soil samples and separating and counting the eggshells we were able to identify these features as preferred sites for oviposition. With flooding, the extant eggs hatch synchronously (Fig. 3). The cycle from larvae to adult proceeds quickly. The larvae feed on leaf litter and become pupae in 5-7 days, with adults then emerging 2 days later (Fig. 4). For the first 24 hrs. after emerging, the females are not interested in a blood meal. They remain close to where they emerged. Mating occurs during this period of quiescence. One can walk through the forest, literally through clouds of mosquitoes (Fig. 5) during these periods and be totally ignored. Afterwards, they migrate out of the mangroves and begin looking for a blood meal. The distance they can migrate is impressive, as much as 50 miles. The shear density of these migratory swarms has, at times, confounded radar.
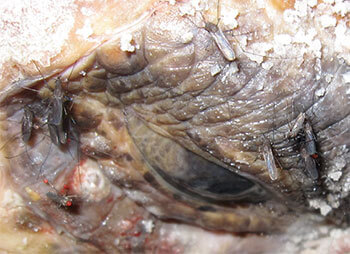
Fig. 1. Taenies feeding near the eye of a nesting
loggerhead turtle. They are most active during
crepuscular periods and at night; however, when
they are out and about during the day, their numbers
will be even more daunting after sunset.
loggerhead turtle. They are most active during
crepuscular periods and at night; however, when
they are out and about during the day, their numbers
will be even more daunting after sunset.

Fig. 4. A raft of pupae in a rain-flooded
mangrove forest. The reflected objects
among the newly emerged adults are their
molts or exuvia. They can be used to identify
mosquitos to species and sex.
mangrove forest. The reflected objects
among the newly emerged adults are their
molts or exuvia. They can be used to identify
mosquitos to species and sex.
Several factors control the size of the broods of adults produced. Eggs gradually accumulate in basin forests over the course the winter and spring dry periods. Monthly high tides and infrequent rainfall produce smaller broods late in the spring. These adults, of course, continue to oviposit in the basin forests and, depending on the frequency and amount of rainfall, more adults are produced. Or, if rainfall is infrequent, more eggs accumulate in the substrate, which, given the right circumstances, can result in stupendous numbers of eggs accumulating. For example, the spring of 1988 was unusually dry. No precipitation occurred through the normally rainy month of June until the last two days of the month when over 10 cm were reported.
This rainfall event flooded local basin forests and triggered the synchronous production of a brood of Taenies whose numbers were almost beyond belief. The basin forests we were studying were so thick with them that without a bug veil we would have had difficulty breathing. A week or so after the onset of this event, three cows in a pasture about 5 km from Rookery Bay National Estuarine Research Reserve died from complications resulting from blood loss. The good news about an event like this is that Taenies in this region are not known to vector human disease, however, they are a vector of the filarial worm Dirofilaria immitis, commonly known as the dog heartworm. But that is little comfort to those unlucky enough to be “carried away” on some warm, humid night by this, “Defender of the Everglades”.

Fig. 5. Newly emerged adults on black mangrove pneumatophores.
At times, there can be so many Taenies perched on
pneumatophores, leaves and twigs that they appear to be fuzzy
or hirsute as a Botanist would say.
At times, there can be so many Taenies perched on
pneumatophores, leaves and twigs that they appear to be fuzzy
or hirsute as a Botanist would say.
References
Addison, D. S., & S. A. Ritchie, (1989). Characterization of preferred habitats of Aedes taeniorhynchus in coastal mangroves habitats. FL HRS Grant #LCNP4. Jacksonville, FL. 106 pp.
Addison, D. S., & Ritchie, S. A. (1993). Cattle fatalities from prolonged exposure to Aedes taeniorhynchus in southwest Florida. Florida Scientist, 65-69. Ritchie, S. A. (1992). Mosquito Control Handbook: Salt Marshes and Mangrove Forests. In Mosquito Control Handbook, 1-9.
Ritchie, S. A., & Addison, D. S. (1992). Oviposition preferences of Aedes taeniorhynchus (Diptera: Culicidae) in Florida mangrove forests. Environmental entomology, 21(4), 737-744
Ritchie, S. A., & Johnson, E. S. (1991). Aedes taeniorhynchus (Diptera: Culicidae) oviposition patterns in a Florida mangrove forest. Journal of medical entomology, 28(4), 496-500.
Helping Sea Turtles Survive for 39 Years
A NON-PROFIT ORGANIZATION
State of Florida Registration Number CH-2841 | Internal Revenue Code 501 (c) (3)
Web Design & Development by Web Expressions, LLC

